IVDR vs IVDD: Understanding the Key Differences
A comprehensive comparison between the new In Vitro Diagnostic Regulation (IVDR) and the former IVD Directive (IVDD), highlighting the most significant changes affecting manufacturers.
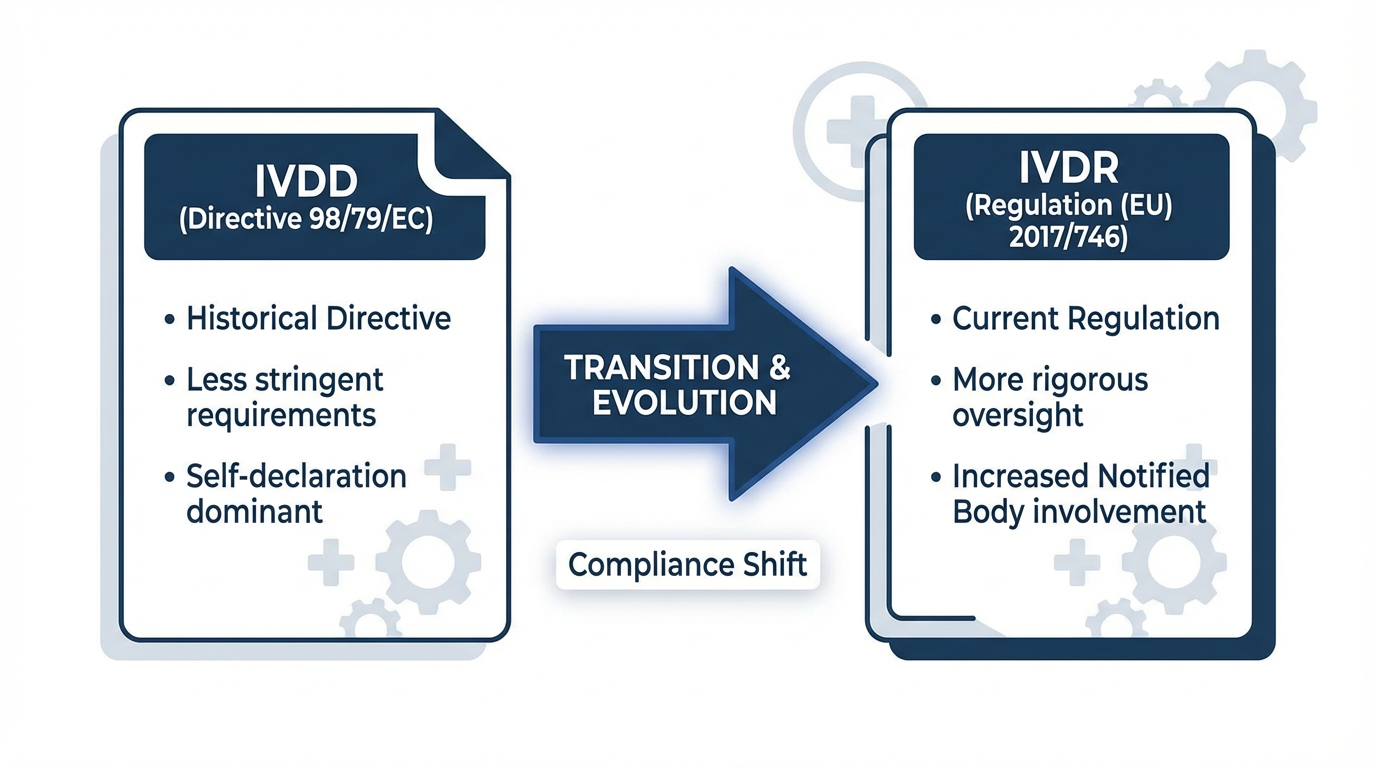
The transition from the In Vitro Diagnostic Directive (98/79/EC) to the In Vitro Diagnostic Regulation (EU) 2017/746 represents the most significant regulatory change for the IVD industry in over two decades.
Classification System Overhaul
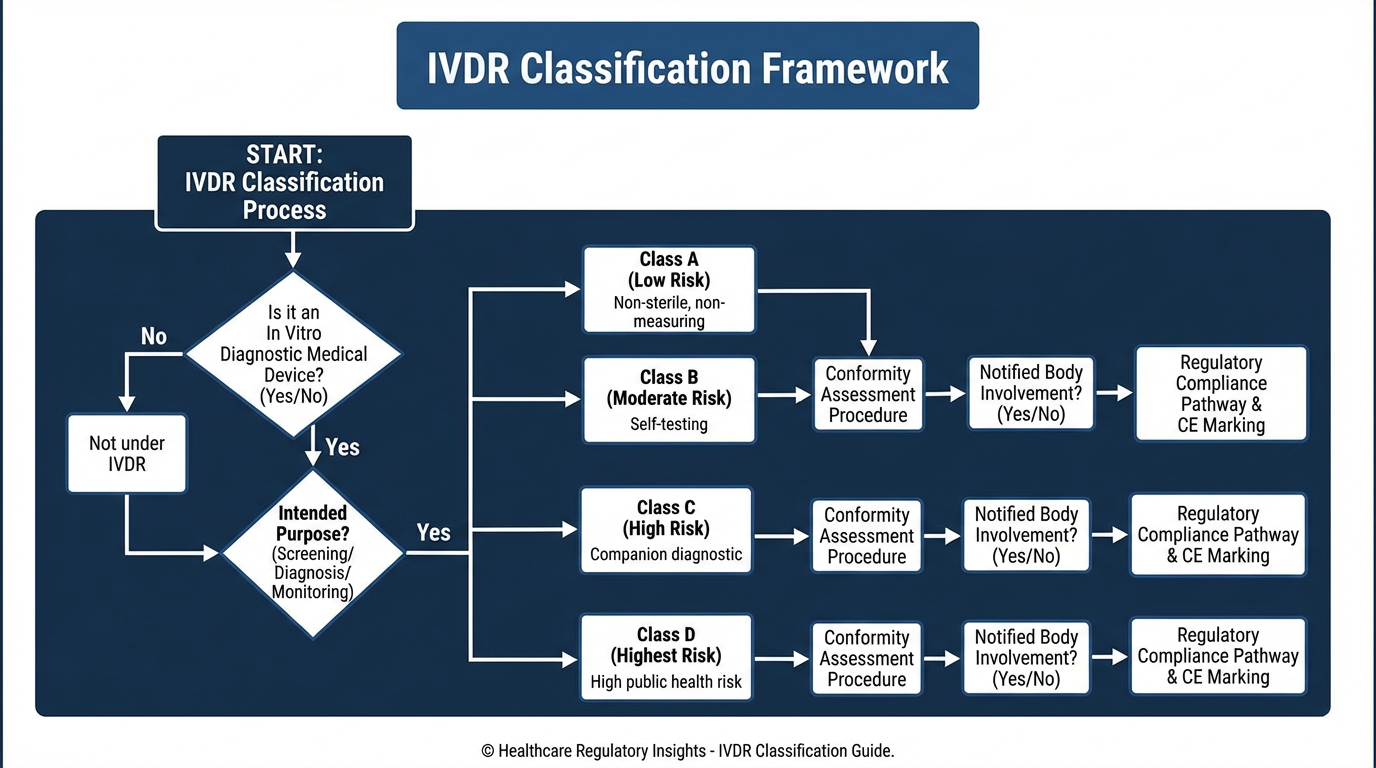
Perhaps the most impactful change is the new risk-based classification system. Under IVDD, most devices were self-certified with only Annex II List A and B devices requiring Notified Body involvement. IVDR introduces four risk classes (A, B, C, D) with significantly more devices requiring Notified Body certification.
Under IVDR:
- Class D: Highest risk (e.g., blood grouping, HIV detection) - requires NB certification
- Class C: High risk (e.g., companion diagnostics, self-testing) - requires NB certification
- Class B: Moderate risk (e.g., many clinical chemistry tests) - requires NB certification
- Class A: Lowest risk - self-certification, but sterile Class A requires NB
Technical Documentation Requirements
IVDR significantly expands technical documentation requirements in Annexes II and III. Key additions include:
- More detailed device description and labeling requirements
- Comprehensive performance evaluation documentation
- Enhanced post-market surveillance documentation
- Risk management aligned with ISO 14971
Performance Evaluation Changes
IVDR introduces much more rigorous performance evaluation requirements under Article 56 and Annex XIII. The concept of "performance evaluation" replaces the simpler performance assessment under IVDD, requiring:
- Scientific validity assessment
- Analytical performance studies
- Clinical performance studies (where applicable)
- Ongoing performance evaluation
Post-Market Surveillance
IVDR establishes comprehensive PMS requirements including Periodic Safety Update Reports (PSURs) for Class C and D devices, and enhanced vigilance reporting obligations.
Transition Timeline
The transition provisions have been extended multiple times, with current deadlines running through 2028 depending on device classification. Manufacturers must carefully plan their transition strategies to meet applicable deadlines.
Get More IVDR Insights
Subscribe to receive expert regulatory updates and compliance tips.
We respect your privacy. Unsubscribe at any time.