Understanding IVDR Device Classification: A Practical Approach
Navigate the IVDR classification rules with this practical guide, including examples for common IVD device types and tips for borderline cases.
IVDR.net Team16 February 20269 min read
Correct classification is fundamental to IVDR compliance as it determines Notified Body involvement and documentation requirements. This guide provides practical classification guidance.
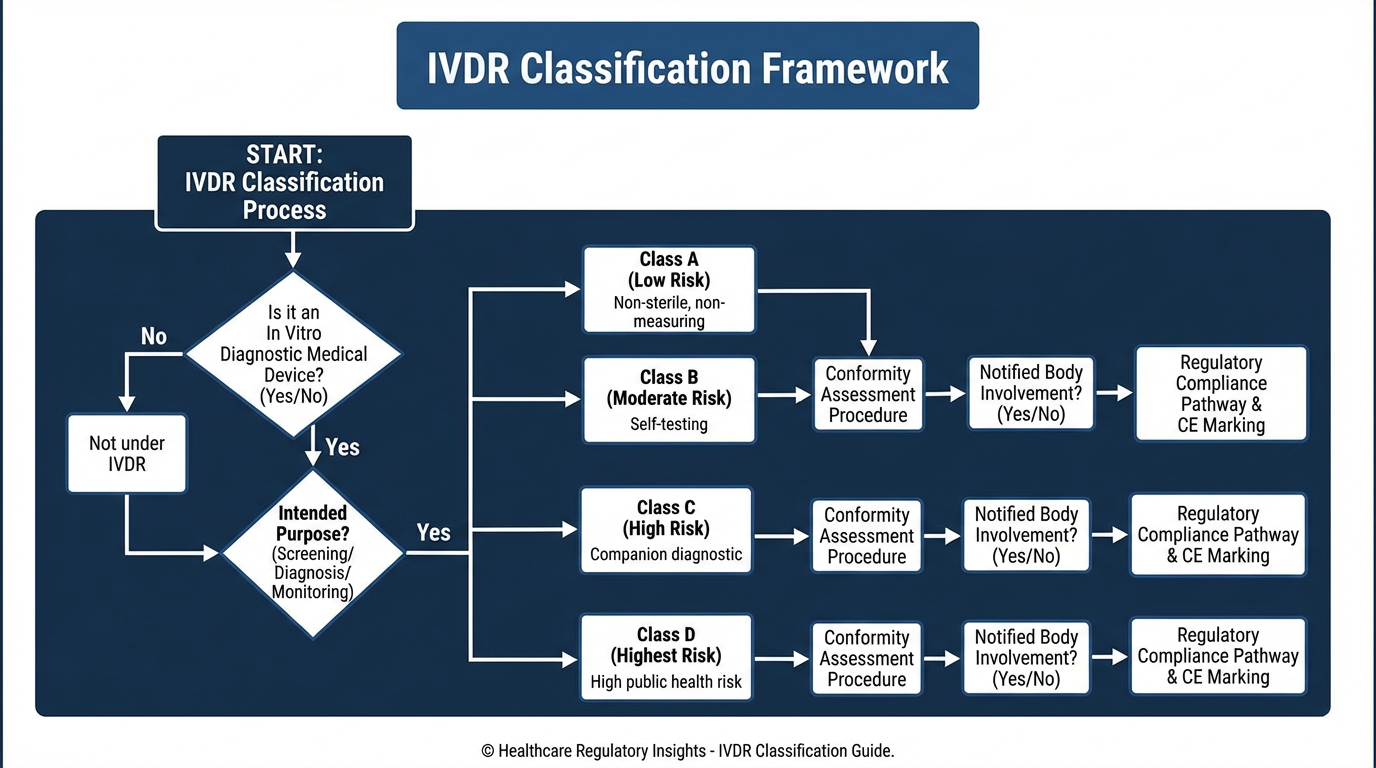
The IVDR Classification System
IVDR establishes four risk classes based on Annex VIII rules:
- Class D: Highest risk - blood typing, transfusion transmissible infections, transplant compatibility
- Class C: High risk - companion diagnostics, genetic testing, self-testing, near-patient testing for life-threatening conditions
- Class B: Moderate risk - most clinical chemistry, general microbiology, pregnancy tests
- Class A: Lowest risk - general laboratory equipment, buffer solutions, specimen containers
Classification Rules Overview
The seven classification rules must be applied in order:
- Rule 1: Devices for detecting transmissible agents in blood/tissues
- Rule 2: Blood grouping devices
- Rule 3: Companion diagnostics, genetic disease detection, contagious disease detection
- Rule 4: Self-testing devices
- Rule 5: Near-patient testing devices
- Rule 6: Instruments
- Rule 7: All other devices (default to Class B, unless A applies)
Common Classification Examples
- HIV screening test: Class D (Rule 1)
- Blood glucose self-test: Class C (Rule 4)
- HLA typing for transplant: Class D (Rule 1)
- Tumor markers for monitoring: Class C (Rule 3)
- General clinical chemistry: Class B (Rule 7)
Documentation Requirements
Always document your classification rationale in your technical file, including the specific rule applied and justification for the intended use interpretation.
Get More IVDR Insights
Subscribe to receive expert regulatory updates and compliance tips.
We respect your privacy. Unsubscribe at any time.