 Regulatory News
Regulatory NewsIVDR Transitional Provisions: Timeline and Strategy Planning
Navigate the extended IVDR transition periods with this comprehensive timeline guide and strategic planning recommendations.

Professional templates, checklists, and expert resources to streamline your EU In Vitro Diagnostic Regulation compliance journey.
Comprehensive resources designed for regulatory affairs professionals, quality managers, and compliance officers.
Professional document templates covering Technical Files, GSPRs, Performance Evaluation Reports, and more. Save weeks of preparation time.
In-depth articles and guides written by regulatory professionals with hands-on IVDR implementation experience.
Stay informed about IVDR amendments, MDCG guidance documents, and transitional provisions affecting your compliance strategy.
Learn from real-world implementation cases and practical insights from the IVD regulatory community.
Join hundreds of compliance teams using our templates and resources.
“The IVDR Technical File template saved us months of work. The structure is comprehensive and aligned perfectly with our Notified Body's expectations.”
Dr. Maria Schmidt
Regulatory Affairs Director
MedTech Solutions GmbH
“Finally, a resource that understands the real challenges of IVDR compliance. The GSPR checklist alone was worth the investment.”
Jean-Pierre Dubois
Quality Manager
DiagnostiQ France
“The Performance Evaluation templates helped us organize our clinical evidence systematically. Our NB audit went smoothly thanks to these resources.”
Anna Kowalski
Compliance Officer
Nordic IVD Labs
Navigate the complex IVDR device classification rules with our comprehensive checklist covering Annex VIII requirements.
We respect your privacy. Unsubscribe at any time.
Expert-Curated Content
Written by regulatory professionals with real-world experience
Regular Updates
Content updated to reflect latest MDCG guidance
Practical Focus
Templates designed for immediate implementation
Stay updated with expert insights on IVDR compliance and implementation.
 Regulatory News
Regulatory NewsNavigate the extended IVDR transition periods with this comprehensive timeline guide and strategic planning recommendations.
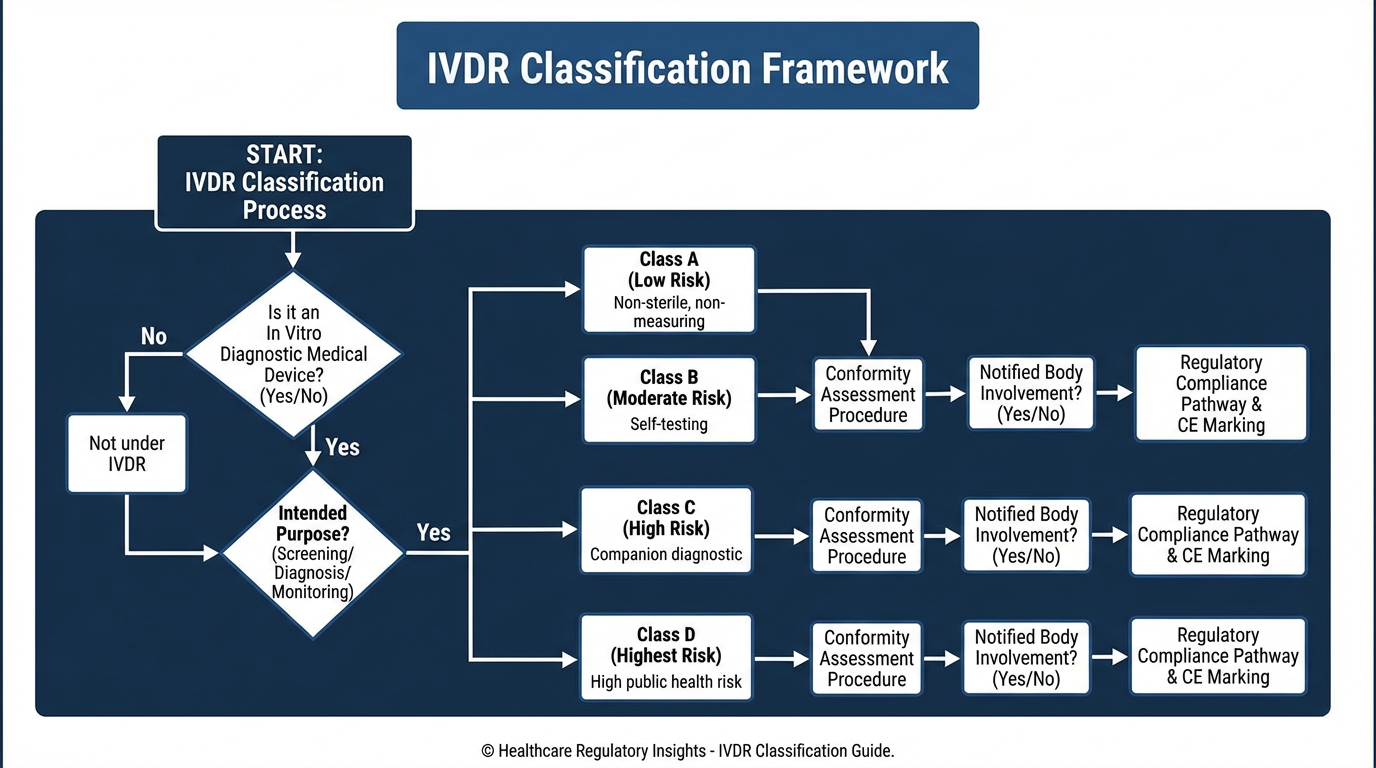 IVDR Fundamentals
IVDR FundamentalsNavigate the IVDR classification rules with this practical guide, including examples for common IVD device types and tips for borderline cases.
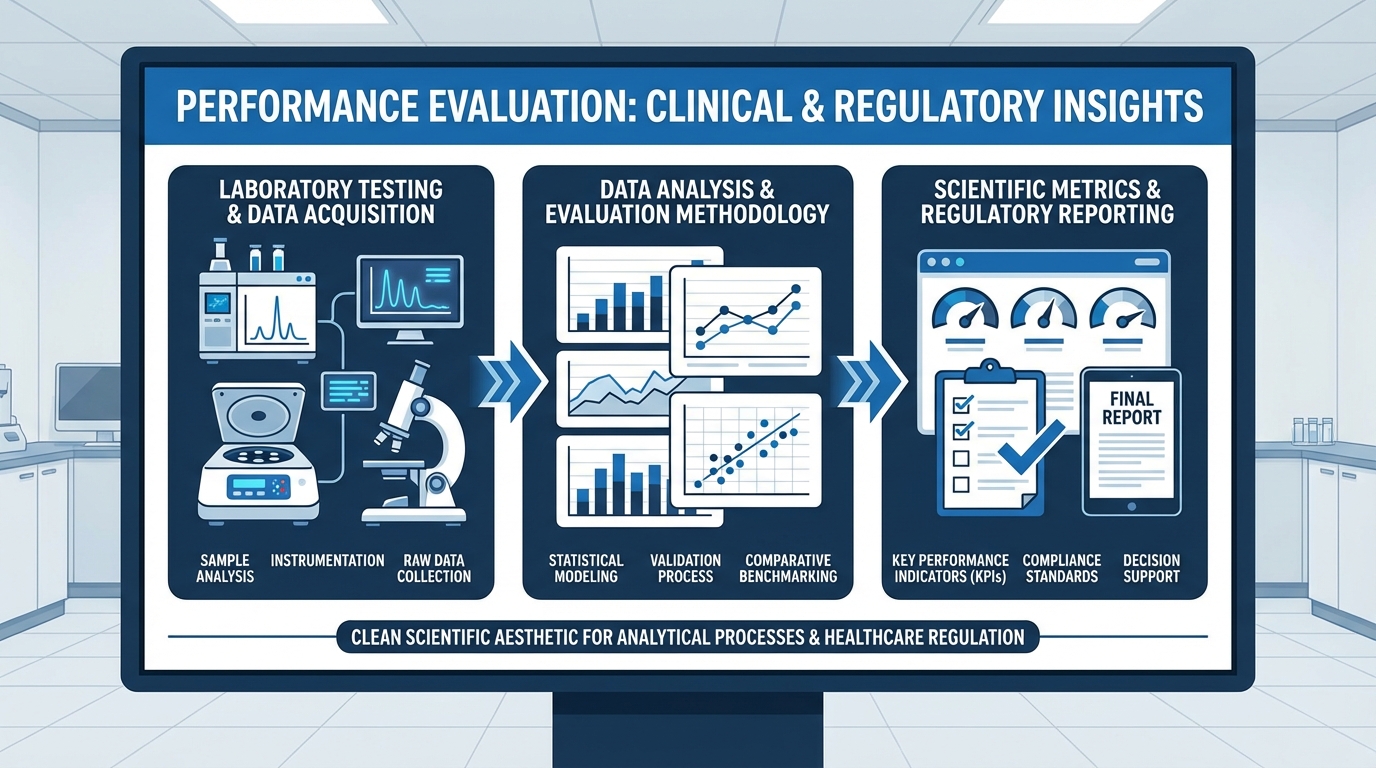 Performance Evaluation
Performance EvaluationComplete guide to planning and executing performance evaluation studies under IVDR, covering scientific validity, analytical performance, and clinical performance requirements.
Our most popular IVDR compliance templates, trusted by regulatory professionals across Europe.